Atmospheric Pressure and Gauge Pressure
Atmospheric Pressure and Gauge Pressure: Overview
This topic covers concepts, such as Absolute Pressure, Gauge Pressure, Hydrostatic Paradox, Atmospheric Pressure, Units Used for Atmospheric Pressure, Measurement of Atmospheric Pressure, Atmospheric Pressure in Terms of Mercury Column, etc.
Important Questions on Atmospheric Pressure and Gauge Pressure
The pressure at the bottom of a tank of water is where is the atmospheric pressure. If the water is drawn out till the level of water is lowered by one-fifth, the pressure at the bottom of the tank will now be
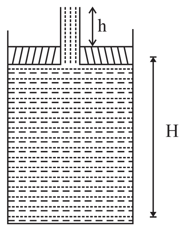
A piston of mass M = 3kg and radius R = 4cm has a hole into which a thin pipe of radius r = 1cm is inserted. The piston can enter a cylinder tightly and without friction, and initially it is at the bottom of the cylinder. 750gm of water is now poured into the pipe so that the piston & pipe are lifted up as shown. Find the height H of water in the cylinder h of water in the pipe
An open U-tube contains mercury. When of water is poured into one of the arms of the tube, then the mercury rise in the other arm from its initial level is
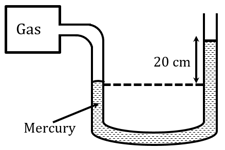
A manometer reads the pressure of a gas in an enclosure as shown in figure. The absolute and gauge pressure of the gas (in of mercury) in the enclosure is
(Take atmospheric pressure = of )
A vessel filled with a liquid of density falls vertically downwards with an acceleration . The gauge pressure at depth below the free surface of liquid is :
The height of the mercury column in a simple barometer is h. As the tube is inclined with the vertical at an angle , the length of the mercury column along the length of the tube will become
The height of mercury column in a simple barometer is . As the tube is inclined with the vertical at an angle , then length of mercury column along the length of the tube will become
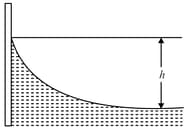
Water of density in a clean aquarium forms a meniscus, as illustrated in the figure. Calculate the difference in height between the centre and the edge of the meniscus. The surface tension of water is .
A -tube of uniform cross-section initially contained a liquid of density and had a length of in the tube. Now another liquid (immiscible with the previous liquid) of density is poured in the tube from one end such that this liquid also fills length in the tube. Find the difference in the levels of the liquid on the two sides of the tubery
A mercury barometer has a cylindrical capillary tube of radius inverted vertically on the mercury in the trough. Atmospheric pressure is of mercury but the level of mercury in the capillary is . The portion of the tube without mercury is in length. Considering that this region is occupied by ideal gasses, the number of moles of the ideal gas will be : (Given: Temperature )
Which instrument is used to measure atmospheric pressure ?
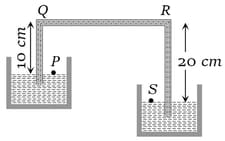
A siphon in use is demonstrated in the following figure. The density of the liquid flowing in siphon is . The pressure difference between the point and will be
With the increasing altitude, gauge pressure:
In the space above the mercury column in a barometer tube the gauge pressure of the vapour is
In the space above the mercury column in a barometer tube, the gauge pressure of the vapour is
Which of the following instrument is used to measure atmospheric pressure?
_____ is an instrument used to measure the atmospheric pressure.
Assuming that the atmosphere has the same density anywhere as at sea level and to be constant . What should be the approximate height of the atmosphere
In a barometer, the mercury level is at sea level. On a hill of height if the ratio of density of to that of air is , the atmospheric pressure on the hill is
The pressure at the bottom of a open water tank where is atmospheric pressure. If water is drawn out till the water level decreases by rd, then pressure at the bottom of the tank will be